Lime Softening
Chemistry
Lime softening involves a relatively complicated series of chemical reactions which will be discussed in depth below. The goal of all of these reactions is to change the calcium and magnesium compounds in water into calcium carbonate and magnesium hydroxide. These are the least soluble calcium and magnesium compounds and thus will settle out of the water at the lowest concentrations. For example, calcium carbonate (which is essentially the same as limestone) will settle out of water at concentrations greater than 40 mg/L.
In order to produce calcium carbonate and magnesium hydroxide, the pH of the water must be raised by the addition of lime. Calcium compounds in water will be removed at a pH of about 9.0 to 9.5 while magnesium compounds require a pH of 10.0 to 10.5. When soda ash is used to remove noncarbonate hardness, an even higher pH is required - 10.0 to 10.5 for calcium compounds and 11.0 to 11.5 for magnesium compounds.
Carbon Dioxide Demand
The first step in lime softening is the addition of lime to water using a typical dry feeder, either volumetric or gravimetric. As in the chlorination process, lime reacts with substances in the water before it can begin softening the water. Carbon dioxide is the primary compound which creates the initial demand for lime. The following reaction occurs, using up carbon dioxide and lime and creating calcium carbonate and water:
Carbon dioxide + Lime → Calcium carbonate + Water
CO2 + Ca(OH)2 → CaCO3 + H2O
The resulting calcium carbonate precipitates out of solution.
When water, especially groundwater, has a high carbon dioxide concentration, the water is often pretreated with aeration before softening begins. Aeration removes the excess carbon dioxide and lowers the lime requirements.
Removal of Carbonate Hardness
Once the carbon dioxide demand has been met, the lime is free to react with and remove carbonate hardness from the water. Calcium compounds react with lime in the reaction shown below.
Calcium bicarbonate + Lime → Calcium carbonate + Water
Ca(HCO3)2 + Ca(OH)2 → 2CaCO3 + 2H2O
We have focussed on calcium bicarbonate since it is the most common calcium compound in water, but other calcium-based hardness compounds have similar reactions. In any case, the calcium carbonate produced is able to precipitate out of solution.
Magnesium compounds have a slightly different reaction. First, magnesium bicarbonate reacts with lime and produces calcium carbonate (which precipitates out of solution) and magnesium carbonate.
Magnesium bicarbonate + Lime → Calcium carbonate + Magnesium carbonate + Water
Mg(HCO3)2 + Ca(OH)2 → CaCO3 + MgCO3 + 2H2O
Then the magnesium carbonate reacts with lime and creates more calcium carbonate and magnesium hydroxide. Both of these compounds are able to precipitate out of water.
Magnesium carbonate + Lime → Calcium carbonate + Magnesium hydroxide
MgCO3 + Ca(OH)2 → CaCO3 + Mg(OH)2
Removal of Noncarbonate Hardness
In many cases, only the carbonate hardness needs to be removed, requiring only the addition of lime. However, if noncarbonate hardness also needs to be removed from water, then soda ash must be added to the water along with lime.
Each noncarbonate hardness compound will have a slightly different reaction. Here, we will consider the reactions of magnesium sulfate. The lime first reacts with the magnesium sulfate, as shown below:
Magnesium sulfate + Lime → Magnesium hydroxide + Calcium sulfate
MgSO4 + Ca(OH)2 → Mg(OH)2 + CaSO4
The resulting compounds are magnesium hydroxide, which will precipitate out of solution, and calcium sulfate. The calcium sulfate then reacts with soda ash:
Calcium sulfate + Soda Ash → Calcium carbonate + Sodium sulfate
CaSO4 + Na2CO3 → CaCO3 + Na2SO4
The calcium carbonate resulting from this reaction will settle out of the water. The sodium sulfate is not a hardness-causing compound, so it can remain in the water without causing problems.
Recarbonation
The reactions which remove carbonate and noncarbonate hardness from water require a high pH and produce water with a high concentration of dissolved lime and calcium carbonate. If allowed to enter the distribution system in this state, the high pH would cause corrosion of pipes and the excess calcium carbonate would precipitate out, causing scale. So the water must be recarbonated, which is the process of stabilizing the water by lowering the pH and precipitating out excess lime and calcium carbonate.
The goal of recarbonation is to produce stable water, which is water in chemical balance, containing the concentration of calcium carbonate in which it will neither tend to precipitate out of the water (causing scale) nor dissolve into the water (causing corrosion.) This goal is usually achieved by pumping carbon dioxide into the water. Excess lime reacts with carbon dioxide in the reaction shown below, producing calcium carbonate:
Lime + Carbon dioxide → Calcium carbonate + Water
Ca(OH)2 + CO2 → CaCO3 + H2O
Recarbonation also lowers the pH, which encourages the precipitation of calcium carbonate and magnesium hydroxide.
Recarbonation may occur in one step, in which the pH is lowered to about 10.4 and carbonate hardness is precipitated out. In some cases, a second recarbonation step is used to lower the pH to 9.8 and encourage yet more precipitation. In either case, the process must be carefully controlled since carbon dioxide can react with calcium carbonate and draw it back into solution as calcium bicarbonate, negating the softening process.
Alternatively, recarbonation can be achieved through the addition of acids such as sulfuric or hydrochloric acids or through polyphosphate addition. These types of recarbonation work differently from carbon dioxide addition.
In The Treatment Process
Equipment Used
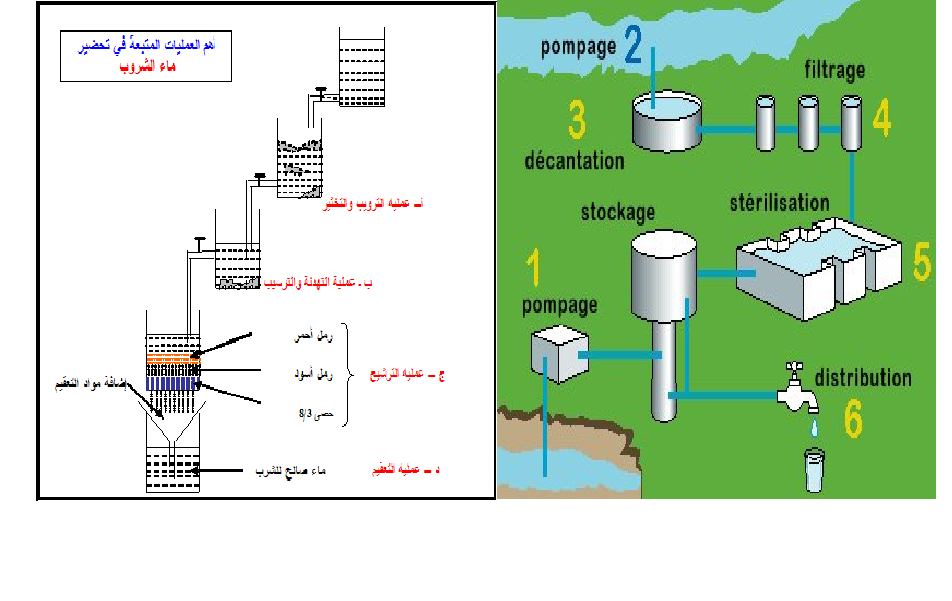
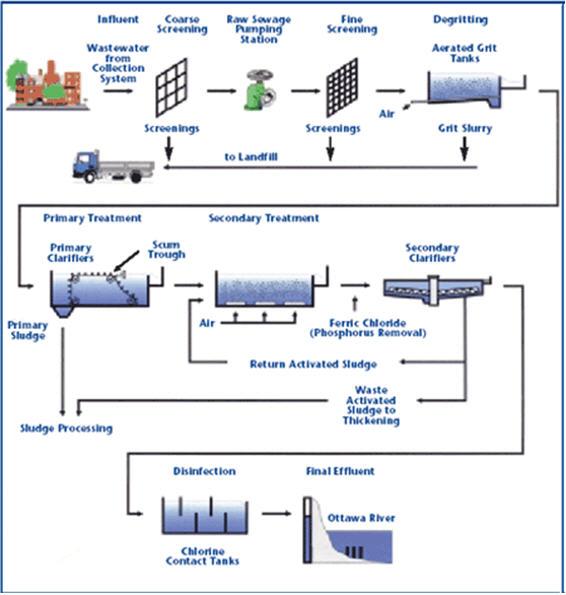
Lime softening uses the equipment already found in most treatment plants for turbidity removal. An overview of the lime treatment process is shown below.
[ltr]

[/ltr]
Sludge
Lime softening produces large quantities of sludge. In fact, for every pound of lime used, about two pounds of sludge are formed.
Lime sludge
The softening process usually requires two sedimentation basins, each with a detention time of 1.5 to 3 hours, to deal with the large quantities of sludge. One sedimentation basin handles the sludge resulting from lime and soda ash softening and the other sedimentation basin deals with the sludge resulting from recarbonation.
Disposal of lime sludge is the same as for sedimentation basin sludge. Landfill disposal is the most common method, although sludge may sometimes be sent to sanitary sewers. Lime sludge has a high pH and has increasingly been disposed of by applying it to agricultural land to increase the pH of acidic soils.
[ltr]
[/ltr]
Monitoring
If softening problems are discovered, the cause usually lies in either chemical feeder malfunctions or source water quality changes. A variety of water characteristics can influence lime-soda ash softening:
- [ltr]Water hardness will determine the quantity of chemicals which must be added to soften the water.[/ltr]
- [ltr]pH influences the chemical reactions in the softening process. A higher pH makes the process more efficient.[/ltr]
- [ltr]Alkalinity determines whether the hardness in the water is carbonate or noncarbonate hardness. [/ltr]
- [ltr]Temperature influences the rate of the reaction and the amount of hardness which the water will hold.[/ltr]
These four water characteristics should be monitored carefully when softening water using lime. In addition, coagulants used to remove turbidity can influence the alkalinity or pH of the water, thus affecting the softening process. After softening, the Langelier Index of the water should be tested to ensure that the water is not corrosive. We will study the Langelier index and corrosive water in more depth in the next lesson.
Softening is especially well-suited to treating groundwater since groundwater characteristics tend to remain relatively constant. Changing water conditions require a great deal of manipulating the softening process to keep it efficient. In addition, the high turbidity found in surface water sometimes requires presedimentation prior to softening.
Chemicals Used in Lime Softening
Types of Lime
The lime used for softening comes in two forms - hydrated lime and quicklime. Both types of lime soften water in the same way, but the equipment required for the two types of lime is different.
Hydrated lime (Ca(OH)2) is also known as calcium hydroxide or slaked lime. Hydrated lime can be added to water as it is without requiring any special equipment, so it is a popular choice for small water treatment plants.
In contrast, quicklime (CaO), also known as calcium oxide or unslaked lime, must be slaked before it is used. Slaking is the process of converting quicklime to hydrated lime by adding water, as shown below:
Calcium oxide + Water → Hydrated lime
CaO + H2O → Ca (OH)2
Slaking requires specialized equipment. The cost of equipment and the operator time required to run the equipment usually make quicklime use uneconomical in small plants. However, since the chemical cost of quicklime is less than the cost of hydrated lime, quicklime is often used in large plants.
The slaking process can also allow a large plant to reuse a large quantity of the lime sludge produced in the softening process. First, the sludge is heated, and the calcium carbonate in the sludge produces calcium oxide:
Calcium carbonate → Calcium oxide + Carbon dioxide
CaCO3 → CaO + CO2
Then the calcium oxide can be slaked and reused in the plant. Reusing lime sludge cuts down on both chemical purchase and sludge disposal costs.
Lime Handling and Storage
Operators should observe safety procedures while handling both hydrated lime and quicklime. Lime dust can be harmful when it comes in contact with the eyes, nose, or mouth, and skin contact can cause burns. As a result, operators should wear goggles and dust masks as well as protective clothing.
Both hydrated lime and quicklime can deteriorate in quality over time while in storage. In addition, storing quicklime can cause safety problems. If quicklime comes in contact with water, it begins to slake, a process which produces a great deal of heat and can cause explosions when uncontrolled. Quicklime should never be stored with alum since the quicklime will absorb water away from the alum and cause an explosion.
Soda Ash
Soda ash (Na2CO3) comes in only one form and does not require any treatment before it is added to the water. Safety issues resemble those for lime handling. Soda ash dust irritates the eyes and mucous membranes of the nose, so the operator should wear protective clothing, goggles, and a dust mask. In addition, areas in which soda ash is used should be equipped with a ventilation system to deal with the dust.
Caustic Soda
Caustic soda (NaOH), also known as sodium hydroxide, can replace soda ash and some of the lime in the treatment process. The treatment process using caustic soda follows the same steps as that of lime-soda ash softening.
First, carbon dioxide reacts with the caustic soda to make sodium carbonate and water.
Carbon dioxide + Caustic soda → Sodium Carbonate + Water
CO2 + 2NaOH → Na2CO3 + H2O
Then the remaining caustic soda can react with calcium bicarbonate and magnesium bicarbonate.
Calcium bicarbonate + Caustic soda → Calcium carbonate + Soda ash + Water
Ca(HCO3)2 + 2NaOH → CaCO3 + Na2CO3 + 2H2O
Magnesium bicarbonate + Caustic soda → Magnesium hydroxide + Soda ash + Water
Mg(HCO3)2 + 4NaOH → Mg(OH)2 + 2Na2CO3 + 2H2O
The caustic soda can also react with magnesium noncarbonate hardness, as shown below. Also note that the reactions between caustic soda and carbonate hardness produced soda ash, which can react with noncarbonate hardness as well.
Magnesium sulfate + Caustic soda → Magnesium hydroxide + Sodium sulfate
MgSO4 + 2NaOH → Mg(OH)2 + Na2SO4
Caustic soda has the advantages of stability in storage, lower sludge formation, and easy handling. However, safety issues still apply. Caustic soda is dangerous to the operator and can cause severe burns to the skin. As a result, rubber gloves, dusk masks, goggles, and a rubber apron should be worn while handling the chemical.






































![[MSF+3.jpg]](https://2img.net/h/4.bp.blogspot.com/_SynCnHGx75g/Shl2T3oVhsI/AAAAAAAAAEY/_7oem5pvQ3I/s1600/MSF%2B3.jpg)








































 [/ltr]
[/ltr]