Admin
Admin

عدد المساهمات : 3762
تاريخ التسجيل : 15/09/2009
العمر : 57
الموقع : مصر
 |  موضوع: معالجة مياه الشرب باستخدام الصوديوم كلوريت موضوع: معالجة مياه الشرب باستخدام الصوديوم كلوريت  الأحد ديسمبر 05, 2010 4:58 pm الأحد ديسمبر 05, 2010 4:58 pm | |
| Sodium chlorite
Sodium chlorite (NaClO2)
Properties
Molecular formula
NaClO2
Molar mass
90.44 g/mol
Appearance white solid
Density
2.5 g/cm3, solid
Melting point
180–200 °C decomp.
Solubility in water
39 g/100 ml (17 °C)
Use
The main application of sodium chlorite is the generation of chlorine dioxide and used for disinfection of a few municipal water treatment plants after put in an acid solution, sodium chlorite breaks down into chlorine dioxide
An advantage in this application, as compared to the more commonly used chlorine, is that One added benefit is that it helps eliminate trihalomethanes (such as chloroform) in drinking water.
When added to a municipal water supply, chlorine dioxide helps control unwanted tastes and odors.
It also aids in the removal of ions like iron andmanganese.
It is produced in large quantities as flakes or a solution from chlorine dioxide and sodium hydroxide.
In its dried state, sodium chlorite (NaClO2) is a white or light yellow-green solid.
The greenish tint comes from trace amounts of CdO2 or iron, which are production residuals.
Sodium chlorite has a molecular weight of 90.44 and decomposes at about 392°F (200°C).
It is generally soluble in water, but its solubility increases as the temperature of the water rises.
Sodium chlorite is a powerful oxidizer that will not explode on percussion.
The anhydrous salt does not absorb water and is stable for up to ten years.
Chemical reagent
In organic synthesis, sodium chlorite is frequently used for the oxidation of aldehydes tocarboxylic acids. The reaction is usually performed in buffered (with monosodium phosphate) solution in the presence of a chlorine scavenger (usually 2-methyl-2-butene).
Water Disinfectant
1. Sodium chlorite, being an alkaline agent, when acidified by using some acid solution, releases chlorine dioxide gas. Sodium chlorite is weakly soluble in water whereas chlorine dioxide is highly water soluble. Chlorine dioxide is a neutral compound of chlorine, which acts as a disinfectant due to its oxidant property, which kills many bacteria and parasites present in water. During the oxidation process chlorine dioxide is reduced to chlorite (ClO2).
2.
Advantage of Using Sodium Chlorite as Disinfectant
3. Sodium chlorite not only kills microorganisms, but it also has many unique features which are not present in other disinfectants releasing chlorine gas for disinfection.
4.
5. It reduces the odor of water without rendering it bitter unlike chlorine compounds for water treatment.
6.
7. It has the added action of controlling iron and manganese ions in the water.
8.
9. The phenolic compounds and hydrogen sulfide released due to some decomposing organic mater in water is also decreased if sodium chlorite is used for water treatment.
10.
Sodium Chlorite for Drinking Water Treatment
11. Chlorine releasing compounds react with organic matter in water and form chloroform like compounds, which are toxic to human body.
12.
13. So, water used for drinking purposes must be treated with sodium chlorite to avoid the toxicity of chlorine.
14.
15. Besides, it is also safe to consume water treated with sodium chlorite.
16.
17. These are the reasons that health-conscious countries and municipalities are increasingly replacing the health-damaging chlorine for the harmless sodium chlorite in treating public water supplies.
It controls microbial contamination in industrial cooling systems and towers.
Food-processing companies use it for washing fruits and vegetables because it is a fungicide.
Raw Materials
The primary raw materials used in the production of sodium chlorite are chlorine dioxide, sodium hydroxide, andhydrogen peroxide.
Chlorine dioxide is a gas at room temperature. Its color is intensely greenish-yellow.
Chlorine dioxide provides the source of chlorine that is converted to sodium chlorite.
In production, it is stored as a liquid solution in glass-lined steel containers.
Sodium hydroxide is a fused solid with a crystalline structure. Also known as caustic soda, it is corrosive to skin and vegetable tissue, causing severe burns.
It is typically produced through the electrolysis of sodium chloride solutions.
Hydrogen peroxide is a colorless liquid that is caustic and bitter to taste. Pure H2 02 is a thick, syrupy liquid that rapidly decomposes into oxygen and water.
In nature, it occurs only in trace amounts in snow or rain. It is naturally generated during lightening storms. It is typically used in dilute solutions during the manufacture of sodium chlorite.
Other materials are typically added to sodium chlorite powders or solutions before they are sold.
Stabilized sodium chlorite solution can be stored for long periods without loss of activity.
When the sodium chlorite is sold as a solid, sodium chloride is often included to make it safer to handle and store.
Manufacture
The free acid, chlorous acid, HClO2, is only stable at low concentrations. Since it cannot be concentrated, it is not a commercial product.
However, the corresponding sodium salt, sodium chlorite, NaClO2 is stable and inexpensive enough to be commercially available.
The corresponding salts of heavy metals (Ag+, Hg+, Tl+, Pb2+, and also Cu2+ and NH4+) decompose explosively with heat or shock.
Sodium chlorite is derived indirectly from sodium chlorate, NaClO3. . It is prepared commercially from the conversion of sodium chlorate (NaClO3) to sodium chlorite (NaClO2).
First, the explosive (only at concentrations greater than 10% in atmosphere) chlorine dioxide, ClO2 is produced by reducing sodium chlorate in a strong acid solution with a suitable reducing agent (for example, sodium sulfite, sulfur dioxide, or hydrochloric acid).
The chlorine dioxide is then absorbed into an alkaline solution and reduced with hydrogen peroxide, H2O2 yielding sodium chlorite.
he Manufacturing Process
While a variety of chlorites are available, sodium chlorite is the only one produced commercially.
It is sold in solution or as a solid.
The technical grade is made up of about 80% sodium chlorite and the rest is sodium chloride.
Large scale production is based on a reaction of chlorine dioxide in a sodium hydroxide solution.
Hydrogen peroxide is also present as the reducing agent.
Sodium chlorite is manufactured in three phases, chlorine dioxide production, sodium chlorite generation, and recovery.
Chlorine dioxide production
• While there are five principal methods for generating chlorine dioxide, the most common is the Hooker R-2 process, which generates chlorine dioxide from sodium chlorate.
•
• During production, solutions of both sodiumchlorate and sodium chloride are pumped into a reaction vessel in approximately equal ratios. Concentrated sulfuric acid is also added to the reaction.
•
• Next, air is bubbled into the bottom of the container to create rapidagitation and dilution of the chlorine dioxide that is produced. During this process, both chlorine dioxide and chlorine gas are created.
•
• These gases are separated out from the reaction vessel.
•
• The chlorine dioxide is separated by being absorbed in a conventional, water chilled tower.
•
• The chlorine gas is passed through separation towers and is picked up as sodium or calcium hypochlorite.
•
• This process produces about a 95% yield of chlorine dioxide.
Sodium chlorite generation
• The chlorine dioxide gas is pumped into a vessel containing a cooled, circulating solution of sodium hydroxide.
•
• These compounds react to form sodium chlorite and sodium chlorate in approximately equal amounts.
•
• Water and oxygen are also generated. To minimize sodium chlorate production, a reducing agent is added. Typically, hydrogen peroxide is used, although sodium peroxide and sodium amalgam may also be employed.
•
• This step is closely monitored because sodium chlorate is highly undesirable in the final product.
Isolation and purification
• Even though steps are taken to minimize its production, sodium chlorate must still be reduced before the sodium chlorite can be isolated.
•
• This is accomplished by adding extra hydrogen peroxide.
•
• The spent reactive solution is then pumped through a fractional crystallization tower to purify the sodium chlorite.
•
• This method takes advantage of the large solubility differences between the chlorite and other related salts that can be formed.
•
• After purification, the sodium chlorite solution is evaporated and tumble dried. If an anhydrous (devoid of water) product is desired, the evaporated powder is mixed with water at lOO°F (38°C).
•
• The solution is saturated and cooled to 77°F (25°C). When this happens, the anhydrous salt spontaneously crystallizes out of the solution.
•
• A rotary drum, steam heated dryer is used to isolated the crystals, resulting in flakes or a fine powder. Occasionally, multiple drying steps are required.
•
• The anhydrous salt can then be converted into powder, granules, or a solution.
•
• Granules are used more often because they are safer, with lower toxic risks and fire hazards, and a homogeneous composition can be created.
•
• Using typical methods, the particle size of the granules can be tightly controlled. Prior to packaging, solid sodium chlorite is mixed with sodium chloride to make it safer to handle.
•
• Solutions are prepared by mixing powdered sodium chlorite with various anticorrosive agents, buffering agents, and surfactants in a mixing vessel. These solutions are used for commercial bleaching processes and can be formulated to be extremely stable.
•
• Depending on the final use, sodium chlorite solution is packaged in plastic containers, drums, tote tanks, andtanker trucks.
•
• Quality Control
To ensure the quality of the sodium chlorite that is produced, the production process is monitored at each stage.
The starting raw materials and the final product are all subjected to a variety of chemical and physical tests to determine that they meet the required specifications.
Some of the commonly tested characteristics include appearance, odor, pH, density, specific gravity, and melting point.
If the final product is a solution, its chemical activity is tested to make sure it has the correct concentration.
For solid granules, particle size is determined and modified if necessary.
Byproducts/Waste
Manufacturing sodium chlorite produces some undesirable byproducts, such as chlorine dioxide, that cannot be released into the immediate environment.
Concentrated fumes of chlorine dioxide are toxic, and cause sickness,appetite loss, and nausea in line operators.
In the production plant, circulation of fresh air is essential. The chlorine dioxide gas is also highly corrosive. For this reason, sodium chlorite solutions must be stored in specially coated containers.
Materials such as glass, porcelain, some plastics, or earthenware are typically used. Titanium is the most resistant metal used today.
Uses of Sodium Chlorite
Sodium chlorite is a white powder that dissolves in water to create an alkaline solution. When mixed with acid, the solution generates chlorine dioxide, a powerful biocide and bleaching agent. In 1967, the Environmental Protection Agency approved liquid chlorine dioxide for treating drinking and wastewater, disinfecting food and food processing equipment and bleaching paper and textile products.
Gaseous chlorine dioxide sterilizes manufacturing and laboratory equipment and cleans rooms. If you use sodium chlorite for these applications, consult a material data safety sheet, because chlorine dioxide can be toxic and corrosive.
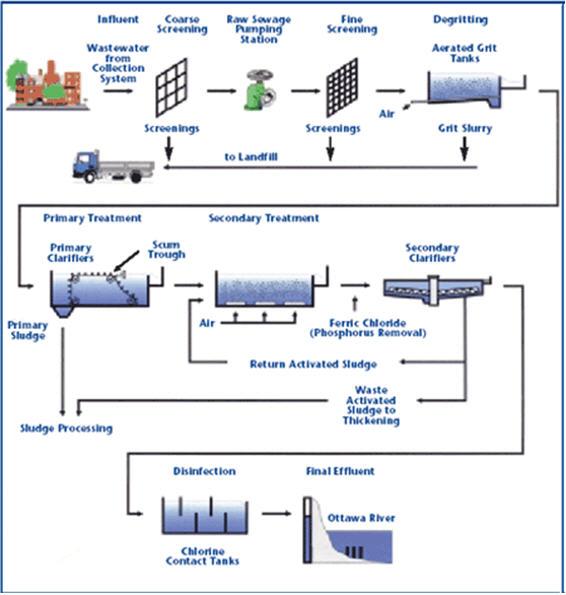
Drinking Water Treatment
Water sterilization by chlorine gas leaves behind trihalomethane byproducts, which are carcinogenic.
Acidified sodium chlorite is preferred to chlorine because it oxidizes cellular components.
The resulting disruption in microbial metabolism produces nontoxic residues that can be removed by filtration or other processes.
Chlorine dioxide from acidified sodium chlorite removes odors and tastes from drinking water by oxidizing sulfur-containing compounds.
Oxidation of iron and manganese in drinking water results in improved color and fewer stains on your sinks and toilets.
Wastewater Treatment
Sodium chlorite is more effective, less toxic and less corrosive than chlorine, according to the Centers for Disease Control and Prevention.
An advantage to generating chlorine dioxide by acidified sodium chlorite is that the gas can be produced on site, eliminating the need for transportation.
Wastewater can contain toxic phenolic agents as well as pathogenic microbes--contaminants that are neutralized by chlorine dioxide.
Decontamination of Surfaces
Chlorine dioxide can remove bacterial slime colonies from industrial and residential plumbing surfaces.
Oxidation of algal growth by chlorine dioxide in industrial cooling towers eliminates the need for costly shutdowns.
Laboratory equipment and clean rooms are sterilized by gaseous chlorine dioxide.
In 2001 and 2002, the Hart Senate Office Building and the U.S. Postal Service Brentwood Processing and Distribution Center in Washington, D.C., were treated with gaseous chlorine dioxide to kill anthrax spores that had passed through these facilities in the mail. The anthrax spores were completely destroyed by the gas, which was generated by activating sodium chlorite
colonel.dr
bahaa badr el-din
| |
|






































![[MSF+3.jpg]](https://2img.net/h/4.bp.blogspot.com/_SynCnHGx75g/Shl2T3oVhsI/AAAAAAAAAEY/_7oem5pvQ3I/s1600/MSF%2B3.jpg)