Admin
Admin

عدد المساهمات : 3762
تاريخ التسجيل : 15/09/2009
العمر : 57
الموقع : مصر
 |  موضوع: Advanced Waste Water Treatment/مراحل المعالجة المتقدمة لمختلف انواع المياه موضوع: Advanced Waste Water Treatment/مراحل المعالجة المتقدمة لمختلف انواع المياه  السبت مارس 10, 2012 11:12 am السبت مارس 10, 2012 11:12 am | |
| Environmental Chemistry and Analysis
Advanced Waste Water Treatment
by
general.dr
bahaa badr
technolab el-bahaa group
Removal Of Suspended Solids 1
Microstraining 2
Coagulation and flocculation 2
Filtration 3
Removal of dissolved solids 3
Ion exchange 4
Reverse osmosis 6
Electrodialysis 7
Removal of nitrogen 8
Phosphate removal (chemical treatment) 9
Phosphate removal (biological treatment) 10
Removal of dissolved organic compounds 10
Adsorption 10
Sludge treatment and disposal 11
Disinfection 13
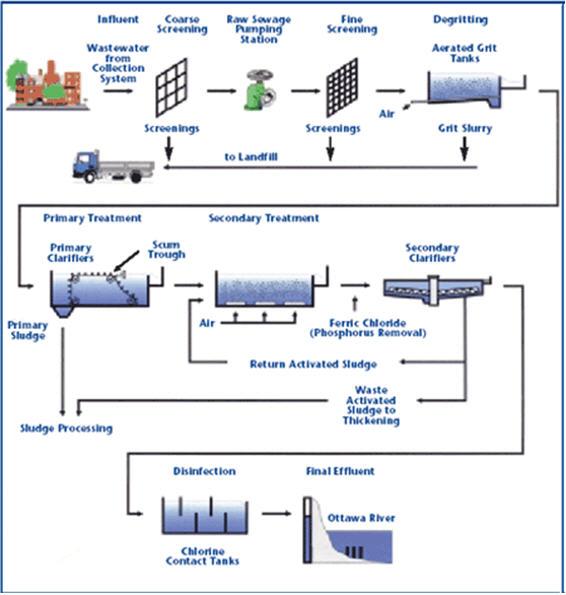
The effluent from a typical secondary treatment plant still contains
20-40 mg/L BOD which may be objectionable in some streams.
Suspended
solids, in addition to contributing to BOD, may settle on the stream bed and
inhibit certain forms of aquatic life.
The BOD if discharged into a stream with low
flow, can cause damage to aquatic life by reducing the dissolved oxygen content.
In addition the secondary effluent contains significant amounts of plant nutrients
and dissolved solids.
If the waste water is of industrial origin, it may also contain
traces of organic chemicals, heavy metals and other contaminants.
Different methods are used in advanced waste treatment to satisfy any of
the several specific goals, which include the removal of :-
(1) suspended solids
(2(BOD
(3) plant nutrients
(4) dissolved solids
(5) toxic substances.
These
methods may be introduced at any stage of the total treatment process as in the
case of industrial waterways or may be used for complete removal of pollutants
after secondary treatment.
Removal Of Suspended Solids:
This treatment implies the removal of those materials that have been
carried over from a secondary treatment settler.
Many methods were proposed of
which two methods were commonly used. The two methods are microstaining
and chemical coagulation followed by settling and mixed media filtration.
Microstraining:
It is a special type of filtration procedure which makes use of filters oven
from stainless steel wires with opening only 60-70 μm across to remove very
small particles.
High flow rates and low back pressures are normally achieved.
Coagulation and flocculation:
The object of coagulation is to alter these particles in such a way as to
allow them to adhere to each other.
Most colloids of interest in water treatment
remain suspended in solution because they have a net negative surface charge
that causes the particles to repel each other.
The intended action of the
coagulant is to neutralise that charge, allowing the particles to come together to
form larger particles that can be more easily removed from the raw water.
The usual coagulant is alum [Al2(SO4)2• 18H2O ], though FeCl3, FeSO4
and other coagulants, such as polyelectrolytes, can be used. Alum when added
to water, the aluminium in this salt hydrolyses by reactions that consume
alkalinity in the water such as:
3+ + − → + +
[Al(H2O)6 ] 3HCO3 Al(OH)3(s) 3CO2 6H2O..............(1)
The gelatinous hydroxide thus formed carries suspended material with it as it
settles.
In addition, however, it is likely that positively charged hydroxyl-bridged
dimers such as
(H2O)4 Al
HO
OH
Al (H2O)4
4+
and higher polymers are formed which interact specifically with colloidal
particles, bringing about coagulation.
Metal ions in coagulants also react with
virus proteins and destroy upto 99% of the virus in water.
Anhydrous ion (III) sulphate can also act as effective coagulant similar to
aluminium sulfate. An advantage with iron (III) sulfate it that it works over a wide
range of pH.
Filtration:
If properly formed, the addition of chemicals for promoting coagulation and
flocculation can remove both suspended and colloidal solids.
After the flocs are
formed, the solution is led to a settling tank where the flocs are allowed to settle.
While most of the flocculated material is removed in the settling tank, some floc
do not settle.
These flocs are removed by the filtration process, which is usually
carried out using beds of porous media such as sand or coal.
The current trend is
to use a mixed -media filter which consists of fine garnet in the bottom layer,
silica sand in the middle layer and coarse coal in the top layer which reduces
clogging.
Removal of dissolved solids:
The dissolved solids are of both organic and inorganic types.
A number of
methods have been investigated for the removal of inorganic constituents from
waste water.
Three methods which are finding wide application in advanced
waste treatment are ion-exchange, electrodialysis and reverse osmosis.
For the
removal of soluble organics from waste water the most commonly used method
is adsorption on activated carbon.
Solvent extraction is also used to recover
certain organic chemicals like phenol an d amines from industrial waste waters.
Ion exchange:
This technique has been used extensively to remove hardness, and iron
and manganese salts in drinking water supplies.
It has also been used selectively
to remove specific impurities and to recover valuable trace metals like chromium,
nickel, copper, lead and cadmium from industrial waste discharges.
The process
takes advantage of the ability of certain natural and synthetic materials to
exchange one of their ions.
A number of naturally occuring minerals have ion exchange properties.
Among them the notable ones are aluminium silicate minerals, which are called
zeolites.
Synthetic zeolites have been prepared using solutions of sodium silicate
and sodium aluminate.
Alternatively synthetic ion-exchange resins composed of
organic polymer with attached functional groups such as (strongly
acidic cation exchange resins), or - COO
-
3 -SO H+
- H+ (weakly acidic cation exchange
resins or -N+(CH3)3OH- (strongly basic anion exchange resins) can be used.
In the water softening process, the hardness producing elements such as
calcium and magnesium are replaced by sodium ions.
A cation exchange resin in
sodium form is normally used.
The water-softening capability of cation exchange
can be seen when sodium ion in the resin is exchanged for calcium ion in
solution.
2Res SO3 Na Ca (Res SO3 )2 Ca 2Na − + + + → − 2+ + +
−
………………….….(2)
(where “Res” represents resin phase)
The product water thus has high sodium content, which is not likely to be
troublesome unless the original water is very hard.
When the exchanger is
saturated, it has to be regenerated to allow reuse of expensive resin.
Regeneration can be achieved by sodium chloride solution which removes Ca2+
and Mg2+ ions from the resin.
2 2
(Res SO3 )2 Ca 2Na 2Cl 2Na (Res SO3 ) Ca 2Cl − + + + + − → + − + + + …….(3)
Since for regeneration large amounts of NaCl has to be used, appreciable
amounts of sodium chloride can be introduced into sewage by this route.
This
problem can be overcome by using weakly acidic cation exchange resin such
ResCOO-H+.
These cation exchangers having -COOH as functional group are
useful for removing alkalinity along with hardness.
Alkalinity is generally
manifested by bicarbonate ion.
This ion is sufficiently basic to neutralise the acid
of weak cation exchange.
Another advantage with these resins is that these can
be regenerated almost stoichiometrically with dilute strong acid, thus avoiding
pollution problem caused by excess NaCl.
This technique has also been
successfully applied to the recovery of chromate from waste water in pigment
manufacturing.
The removal of inorganic solute is essential for complete water recycling.
The effluent from secondary waste treatment contains 300-400 mg/L more
dissolved inorganic material than does municipal water.
The removal of these
bulk inorganics can be efficiently done by reverse osmosis and electrodialysis .
Reverse osmosis:
In the reverse osmosis process, demineralisation water is produced by
forcing water through semipermeable membranes at high pressure.
In ordinary
osmosis, if a vessel is divided by a semipermeable membrane (one that is
permeable to water but not the dissolved material), and one compartment is filled
with water and other with concentrated salt solution, water diffused through the
membrane towards the compartment containing salt solution until the difference
in water levels on the two sides of the membrane creates a sufficient pressure to
counteract the original water flow.
The difference in levels represents the osmotic
pressure of the solution .
Pure
water
Salt
solution
Osmosis
membrane
The process can be reversed by applying sufficient pressure to the
concentrated solution to overcome the osmotic pressure force the net flow of
water through the membrane towards the dilute phase.
The solute concentration
(impurity) builds up on one side of the membrane while relatively pure water
passes through the membrane .
In order to obtain adequate solvent (water) flux
through the membrane, pressures of the order of 4000 to 7000 kN/m2 are
required.
Pressure
membrane
Pure Salt
water solution
Reverse Osmosis
Electrodialysis:
Electrodialysis uses ion-selective membranes and an electrical potential
difference to separate anions and cations in solution.
+ - + - + -
- - -
-
-
+ + +
+
Electrode
membrane
passes only
ions
membrane
passes only
+ ions
Electrode
deionised water
salt solution
Electrodialysis cell
In the past electrodialysis was most often used for purifying brackish
water, but it is now finding a role in hazardous waste treatment. Metal salts from
plating rinses are sometimes removed in this way.
two types of membranes (anionic and cationic) are
arranged alternatively to form many compartments between the electrodes
placed at the two ends.
When the voltage is applied across the cell containing
mineralised water, the anions migrate to the positive electrode and the cations
migrate to the negative electrode.
This causes solution in alternate
compartments to become more concentrated while that in the remaining
becomes more dilute.
The electric power requirement is proportional to the
number of ions removed from the water.
In the electrodialysis process, organic molecules are not removed and
they can collect on and clog the membranes.
Another disadvantage of this
method is that it still leaves concentrated waste water to be disposed of by some
appropriate scheme.
The process does not require any chemical additives and
has low energy requirements and as such it can be an economically feasible
means of demineralisation.
Removal of nitrogen:
Nitrogen compounds may be removed in waste water in two ways.
Even after
secondary treatment, most of nitrogen exists as ammonia. Increasing the pH
produces the reaction,
NH4 OH NH3 H O + − + → ↑+2
...............................................................(4)
Much of the dissolved ammonia gas may then be expelled from the water
into the atmosphere.
The ammonium ion in the waste water may also be oxidised
to nitrate by bacteria like nitrobacter and nitrosomonas, in a process called
nitrification.
nitrosomonas
2NH4 3O2 2NO2 2H2O 4H + − + ⎯⎯⎯⎯⎯⎯→ + ++
3 −
2
............................(5)
nitrobacter
2NO2 O2 2NO − + ⎯⎯⎯⎯⎯→ .......................................... ....... .....(6)
These reactions are slow and require long retention times in the aeration
tank as well as sufficient DO.
If the flow rate is too high, the slow-growing
microorganisms are washed out of the aeration tank.
Once the ammonia has been oxidised to nitrate, it may be reduced by
anaerobic bacteria like pseudomonas.
This denitrification requires a source of
carbon and methanol is often used for that purpose.
6NO3 2CH3OH 6NO2 2CO2 4H O − + → − + ↑ + ..................................(7)
6NO2− + 3CH3OH →3N2 ↑ +3CO2 ↑ +3H2O+ 6OH− .......................(8)
Phosphate removal (chemical treatment):
Phosphate may be removed chemically or biologically.
The most popular
chemical methods use lime, Ca(OH)2 and alum, Al2(SO4)3.
Under alkaline
conditions, the calcium will combine with phosphate to form calcium
hydroxyapatite, a white insoluble precipitate that is settled out and removed from
waste water.
Insoluble calcium carbonate is also formed and removed.
5 Ca(OH)2 3HPO4 Ca5OH(PO4 )3 3H2O 6OH + − → ↓ + + − .................(9)
The aluminium ion from alum precipitates as very slightly soluble
aluminium phosphate,
3 3
Al PO4 AlPO + + − → 4 ↓ ..................................................................(10)
and also forms aluminium hydroxide.
3
Al 3OH Al(OH)3 + + − → ↓ ................................................................(11)
which forms sticky flocs that help to settle out phosphates.
Phosphate removal (biological treatment)
Biological phosphorous removal does not require the addition of
chemicals.
In this process, the aeration tank in the activated sludge system is
subdivided into zones, some of which are not aerated. In these zones, the
aerobic microorganisms become solely stressed because of the lack of oxygen.
If
these microorganisms are then transferred to an aerated zone, they try to make
up for lost time and assimilate organic matter (as well as phosphorous) at a rate
much higher than they ordinarily would.
Once the microorganisms have adsorbed
the phosphorous, they are removed as waste activated sludge, thus carrying with
them high concentrations of phosphorous. Using such sequencing of nonaerated
and aerated zones, it is possible to remove as much as 90% of the phosphorous.
Removal of dissolved organic compounds:
Adsorption:
One of the most commonly used techniques for removing organics involves
the process of adsorption, which is the physical adhesion of chemicals on to the
surface of the solid.
The effectiveness of the adsorbent is directly related to the
amount of surface area available to attract the particles of contaminant.
The most
commonly used adsorbent is a very porous matrix of granular activated carbon,
which has an enormous surface area (~ 1000 m2/g). Adsorption on activated
carbon is perhaps the most economical and technically attractive method
available for removing soluble organics such as phenols, chlorinated
hydrocarbons, surfactants, and colour and odour producing substances from
waste water.
Granular activated carbon treatment systems consist of a series of large
vessels partially filled with adsorbent.
Contaminated water enters the top of each
vessel, trickles down through granulated activated carbon, and is released at the
bottom.
After a period of time, the carbon filter becomes clogged with adsorbed
contaminants and must be either replaced or regenerated.
Regeneration of the
carbon is accomplished by heating it to 950oC in a steam air atmosphere.
This
process oxidises surface, with an approximately 10% loss of carbon.
Synthetic organic polymers such as Amberlite XAD-4 have hydrophobic
surfaces and are quite useful in removing relatively insoluble organic compounds
such as chlorinated pesticides.
These absorbents are readily regenerated by
solvents such as isopropanol and acetone.
Sludge treatment and disposal:
Both primary and secondary sewage treatments involve settling of
particulate matter, and thus produce sludge.
The concentration of solids in the
primary sewage sludge is about 5%; the activated sludge contains about 1%; and
the sludge from trickling filters has about 2% solids.
Thus the sludge is
composed of almost entirely of water and volume reduction is the key to
economic disposal.
In addition to reducing its high water content, the sludge must
be stabilised so that its biological activity and tendency towards putrefaction are
reduced drastically.
The sludge is concentrated by gravity settling and floatation.
After
concentration the sludge is subjected to anaerobic digestion in a digester in
which the organic content of the sludge decomposes to give mainly methane and
carbondioxide and at the same time the bound water is released from the sludge.
The sludge is then conditioned to improve its dewatering characteristics by
adding chemicals like iron salts and polyelectrolytes.
These chemicals bind the
sludge particles together and encourage the release of water.
The sludge is then
heated under pressure and after a period of time the gel structure of the sludge
breaks down so that the water is released.
The thickened sludge is then
dewatered for efficient handling and disposal.
The dewatering is accomplished by
mechanical methods, the most common being centrifugation and filtration. The
dewatered sludge is then subjected to oxidation to reduce the organic content,
with the consequent destruction of bacteria and a significant reduction in their
volumes.
Incineration and wet oxidation are the two common methods employed
for oxidation.
Several methods are employed for the ultimate disposal of sludge.
The
wet digested sludge may be sprayed on to a cropland where it functions as
fertiliser.
Dried sludge may be used a land fill or soil conditioner.
Wet or partially
dewatered sludge or ash from incineration may be transported from the shore to
dumping grounds at sea.
The potential drawback to the use of sewage sludge as
fertiliser in agricultural fields is the presence of both organic and inorganic toxic
compounds.
The former compounds are oxidation-resistant organic substances,
such as organochlorine species which become bound in the organic matrix of the
sludge.
The inorganic toxicants are represented by heavy metals, mainly arsenic,
cadmium , lead, mercury and zinc.
These metals can be taken up by crops and
introduced into the food chains or leached to the ground water.
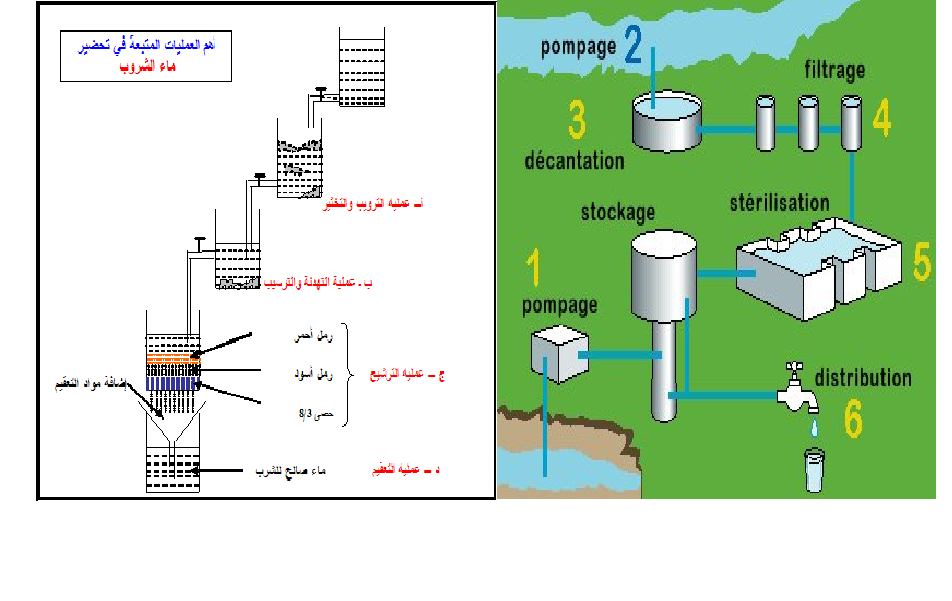
Disinfection:
Disinfection, using chemical and physical methods is the final step in
drinking water purification.
The finished water is disinfected often with chlorine.
It
kills the remaining microorganisms in the water, some of which will be
pathogenic. It is a very efficient oxidising, bleaching and disinfecting agent.
In
water chlorine reacts as follows:
Cl2 H2O H Cl HOCl + ←⎯⎯⎯⎯→ + + − + …………………………………………...(12)
The hypochlorous acid (HOCl) is the prime disinfecting agent.
Its
dissociation in pH dependent yielding less effective hypochlorite ions (OCl-) at
higher pH values:
HOCl←⎯⎯⎯⎯→H+ +OCl−…………………………………………………….…(13)
Together, HOCl and OCl- are called the free available chlorine.
A principal advantage of chlorination over other forms of disinfection is
that a chlorine residual is created that can protect the treated water after leaving
the treatment plant.
This guards against possible contamination that might occur
in water distribution system. To increase the lifetime of the residual, some
systems add ammonia to the treated water, forming chloramines.
NH4 HOCl NH2Cl(monochloramine) H O H + + ⎯⎯→ + 2 + +
+ 2
+ 2
………………………..(14)
………………………...………(15)
………………………..............(16)
NH2Cl +HOCl⎯⎯→NHCl2(dichloramine) H O
NHCl2 +HOCl⎯⎯→NCl3(trichloramine) H O
Chloramines, although they are less effective as oxidants than HOCl, are
more persistent.
Residual chlorine that exists as chloramine is referred to as
combined available chlorine.
Chlorine may have adverse secondary effects.
It has the potential to
combine with trace amounts of organic substances to form trihalomethanes
(THMs) such as the carcinogen chloroform.
Some studies have shown an
association between bladder and rectal cancer and consumption of chlorinated
drinking water.
One approach to reducing THMs is to remove more of the
organics before any chlorination takes place, which can be accomplished by
adsorption on activated carbon.
The problem faced with the formation of THMs has spurred interest in
alternatives to chlorination as the preferred method of disinfection.
Alternative
disinfectants include chlorine dioxide and ozone. Chlorine dioxide (ClO2) is a
potent bactericide and viricide and it does not form a residual capable of
protecting water in the distribution system.
However, there is concern for certain
toxic chlorate and chlorite substances that it may create, and it is a very costly
method of disinfection. Ozonation involves the passage of ozone (O3) through
water.
Ozone is a very powerful disinfectant that is even more effective against
cysts and viruses than chlorine, and it has the added advantage of having no
taste or odour problems. Unfortunately, the disinfective power of ozone is limited
by its relatively low solubility in water.
| |
|






































![[MSF+3.jpg]](https://2img.net/h/4.bp.blogspot.com/_SynCnHGx75g/Shl2T3oVhsI/AAAAAAAAAEY/_7oem5pvQ3I/s1600/MSF%2B3.jpg)