Admin
Admin

عدد المساهمات : 3762
تاريخ التسجيل : 15/09/2009
العمر : 57
الموقع : مصر
 |  موضوع: Lمبادئ عملية التجلط والتطويف فى معالجة المياه/ Coagulation and Flocculation موضوع: Lمبادئ عملية التجلط والتطويف فى معالجة المياه/ Coagulation and Flocculation  الأربعاء مارس 21, 2012 11:23 am الأربعاء مارس 21, 2012 11:23 am | |
|
Coagulation and Flocculation
BY
TECHNOLAB EL-BAHAA GROUP
GENERAL.DR
BAHAA BADR
Objective
In this lesson we will answer the following questions:
How do coagulation and flocculation fit into the water treatment process?
Which chemical principles influence coagulation and flocculation?
Which chemicals are used in coagulation?
What factors influence coagulation and flocculation?
Lecture
Overview of the Process
Location in the Treatment Plant
After the source water has been screened and has passed through the optional steps of pre-chlorination and aeration, it is ready for coagulation and flocculation.
In theory and at the chemical level, coagulation and flocculation is a three step process, consisting of flash mixing, coagulation, and flocculation.
However, in practice in the treatment plant, there are only two steps in the coagulation/flocculation process - the water first flows into the flash mix chamber, and then enters the flocculation basin.
In this lesson, we will primarily be concerned with the theory behind coagulation/flocculation.
Purpose
The primary purpose of the coagulation/flocculation process is the removal of turbidity from the water.
Turbidity is a cloudy appearance of water caused by small particles suspended therein. Water with little or no turbidity will be clear.
Turbidity is not only an aesthetic problem in water. Water with a high turbidity can be very difficult or impossible to properly disinfect.
As a result, the maximum allowable level of turbidity in water is 0.5 NTU, while the recommended level is about 0.1 NTU. (NTU, or TU, stands for nephelometric turbidity units, a measurement of the turbidity of water.)
In addition to removing turbidity from the water, coagulation and flocculation is beneficial in other ways.
The process removes many bacteria which are suspended in the water and can be used to remove color from the water.
Turbidity and color are much more common in surface water than in groundwater.
As surface water flows over the ground to streams, through streams, and then through rivers, the water picks up a large quantity of particles.
As a result, while aeration is more commonly required for groundwater, treatment involving coagulation and flocculation is typical of surface water.
Three Steps
As I mentioned above, the chemistry of coagulation/flocculation consists of three processes - flash mix, coagulation, and flocculation.
Each of these processes is briefly explained below.
In the flash mixer, coagulant chemicals are added to the water and the water is mixed quickly and violently.
The purpose of this step is to evenly distribute the chemicals through the water.
Flash mixing typically lasts a minute or less. If the water is mixed for less than thirty seconds, then the chemicals will not be properly mixed into the water.
However, if the water is mixed for more than sixty seconds, then the mixer blades will shear the newly forming floc back into small particles.
After flash mixing, coagulation occurs. During coagulation, the coagulant chemicals neutralize the electrical charges of the fine particles in the water, allowing the particles to come closer together and form large clumps.
You may already be familiar with the process of coagulation from cooking.
You can see coagulation occurring when preparing gelatin (jello) or when cooking an egg white.
The final step is flocculation. During flocculation, a process of gentle mixing brings the fine particles formed by coagulation into contact with each other.
Flocculation typically lasts for about thirty to forty-five minutes.
The flocculation basin often has a number of compartments with decreasing mixing speeds as the water advances through the basin.
This compartmentalized chamber allows increasingly large floc to form without being broken apart by the mixing blades.
Floc
The end product of a well-regulated coagulation/flocculation process is water in which the majority of the turbidity has been collected into floc, clumps of bacteria and particulate impurities that have come together and formed a cluster.
The floc will then settle out in the sedimentation basin, with remaining floc being removed in the filter.
Floc
The best floc size is 0.1 to 3 mm. Larger floc does not settle as well and is more subject to breakup in the flocculation basin. Smaller floc also may not settle.
Part 2: Chemistry
Introduction
Why do we need such a complex process to remove particles from water?
Some particles would settle out of the water on their own, given enough time.
But other particles would resist settling for days or months due to small particle size and to electrical charges between the particles.
We will consider the chemical processes which prevent and aid settling below.
But first, we will list the three types of objects which can be found in water.
Particles in Water
There are three types of objects which can be found in water. In order from smallest to largest, these objects are chemicals in solution, colloidal solids, and suspended solids.
Coagulation/flocculation will remove colloidal and suspended solids from water.
Chemicals in solution have been completely dissolved in the water.
They are electrically charged and can interact with the water, so they are completely stable and will never settle out of the water.
Chemicals in solution are not visible, either using the naked eye or using a microscope, and are less than 1 Mu in size.
(A Mu, or millimicron, is equal to 0.000000039 inches.) An example of a chemical in solution is sugar in water.
Colloidal solids, also known as nonsettleable solids, do not dissolve in water although they are electrically charged.
Still, the particles are so small that they will not settle out of the water even after several years and they cannot be removed by filtration alone.
Colloidal solids range between 1 and 500 Mu in size and can be seen only with a high-powered microscope.
Examples include bacteria, fine clays, and silts. Colloidal solids often cause colored water, such as the "tea color" of swamp water.
Finally, suspended, or settleable, solids will settle out of water over time, though this may be so slow that it is impractical to merely allow the particles to settle out in a water treatment plant.
The particles are more than 1,000 Mu in size and can be seen with a microscope or, sometimes, with the naked eye. Examples of suspended solids include sand and heavy silts.
Electrical Charges
The chemistry of coagulation and flocculation is primarily based on electricity.
Electricity is the behavior of negative and positively charged particles due to their attraction and repulsion.
Like charges (two negatively charged particles or two positively charged particles) repel each other while opposite charges (a positively charged particle and a negatively charged particle) attract.
Negative charges make particles repel each other.
Negatively charged particles repel each other due to electricity.
Most particles dissolved in water have a negative charge, so they tend to repel each other.
As a result, they stay dispersed and dissolved or colloidal in the water, as shown above.
The purpose of most coagulant chemicals is to neutralize the negative charges on the turbidity particles to prevent those particles from repelling each other.
The amount of coagulant which should be added to the water will depend on the zeta potential, a measurement of the magnitude of electrical charge surrounding the colloidal particles.
You can think of the zeta potential as the amount of repulsive force which keeps the particles in the water.
If the zeta potential is large, then more coagulants will be needed.
Coagulants tend to be positively charged.
Due to their positive charge, they are attracted to the negative particles in the water, as shown below.
Coagulants attract to the particles in water.
Positively charged coagulants attract to negatively
charged particles due to electricity.
The combination of positive and negative charge results in a neutral, or lack, of charge. As a result, the particles no longer repel each other.
The next force which will affect the particles is known as van der Waal's forces. Van der Waal's forces refer to the tendency of particles in nature to attract each other weakly if they have no charge.
Van der Waal's forces cause the particles to drift together.
Neutrally charged particles attract due to van der Waal's forces.
Once the particles in water are not repelling each other, van der Waal's forces make the particles drift toward each other and join together into a group.
When enough particles have joined together, they become floc and will settle out of the water.
Particles join together into floc.
Particles and coagulants join
together into floc.
Part3: Coagulant Chemicals
Types of Coagulants
Coagulant chemicals come in two main types - primary coagulants and coagulant aids.
Primary coagulants neutralize the electrical charges of particles in the water which causes the particles to clump together.
Coagulant aids add density to slow-settling flocs and add toughness to the flocs so that they will not break up during the mixing and settling processes.
Primary coagulants are always used in the coagulation/flocculation process. Coagulant aids, in contrast, are not always required and are generally used to reduce flocculation time.
Chemically, coagulant chemicals are either metallic salts (such as alum) or polymers.
Polymers are man-made organic compounds made up of a long chain of smaller molecules.
Polymers can be either cationic (positively charged), anionic (negatively charged), or nonionic (neutrally charged.)
The table below shows many of the common coagulant chemicals and lists whether they are used as primary coagulants or as coagulant aids.
Different sources of water need different coagulants, but the most commonly used are alum and ferric sulfate.
Chemical Name
Chemical Formula
Primary Coagulant
Coagulant Aid
Aluminum sulfate (Alum)
Al2(SO4)3 · 14 H2O
X
Ferrous sulfate
FeSO4 · 7 H2O
X
Ferric sulfate
Fe2(SO4)3 · 9 H2O
X
Ferric chloride
FeCl3 · 6 H2O
X
Cationic polymer
Various
X
X
Calcium hydroxide (Lime)
Ca(OH)2
X*
X
Calcium oxide (Quicklime)
CaO
X*
X
Sodium aluminate
Na2Al2O4
X*
X
Bentonite
Clay
X
Calcium carbonate
CaCO3
X
Sodium silicate
Na2SiO3
X
Anionic polymer
Various
X
Nonionic polymer
Various
X
*Used as a primary coagulant only in water softening processes.
Alum
There are a variety of primary coagulants which can be used in a water treatment plant.
One of the earliest, and still the most extensively used, is aluminum sulfate, also known as alum.
Alum can be bought in liquid form with a concentration of 8.3%, or in dry form with a concentration of 17%. When alum is added to water, it reacts with the water and results in positively charged ions.
Coagulant Aids
Nearly all coagulant aids are very expensive, so care must be taken to use the proper amount of these chemicals.
In many cases, coagulant aids are not required during the normal operation of the treatment plant, but are used during emergency treatment of water which has not been adequately treated in the flocculation and sedimentation basin.
A couple of coagulant aids will be considered below.
Lime is a coagulant aid used to increase the alkalinity of the water.
The increase in alkalinity results in an increase in ions (electrically charged particles) in the water, some of which are positively charged.
These positively charged particles attract the colloidal particles in the water, forming floc.
Bentonite is a type of clay used as a weighting agent in water high in color and low in turbidity and mineral content.
This type of water usually would not form floc large enough to settle out of the water.
The bentonite joins with the small floc, making the floc heavier and thus making it settle more quickly.
Factors Influencing Coagulation
Introduction
In a well-run water treatment plant, adjustments are often necessary in order to maximize the coagulation/flocculation process.
These adjustments are a reaction to changes in the raw water entering the plant.
Coagulation will be affected by changes in the water's pH, alkalinity, temperature, time, velocity and zeta potential.
The effectiveness of a coagulant is generally pH dependent. Water with a color will coagulate better at low pH (4.4-6) with alum.
Alkalinity is needed to provide anions, such as (OH) for forming insoluble compounds to precipitate them out.
It could be naturally present in the water or needed to be added as hydroxides, carbonates, or bicarbonates.
Generally 1 part alum uses 0.5 parts alkalinity for proper coagulation.
The higher the temperature, the faster the reaction, and the more effective is the coagulation.
Winter temperature will slow down the reaction rate, which can be helped by an extended detention time.
Mostly, it is naturally provided due to lower water demand in winter.
Time is an important factor as well. Proper mixing and detention times are very important to coagulation.
The higher velocity causes the shearing or breaking of floc particles, and lower velocity will let them settle in the flocculation basins.
Velocity around 1 ft/sec in the flocculation basins should be maintained.
Zeta potential is the charge at the boundary of the colloidal turbidity particle and the surrounding water.
The higher the charge the more is the repulsion between the turbidity particles, less the coagulation, and vice versa.
Higher zeta potential requires the higher coagulant dose. An effective coagulation is aimed at reducing zeta potential charge to almost 0.
Coagulant
The proper type and concentration of coagulant are essential to the coagulation process.
The coagulant choice will depend on the conditions at the plant.
The concentration of coagulant also depends on the water conditions, and a jar test can be used to determine the correct concentration to use at any given time.
Coagulants are usually fed into the water using a gravimetric feeder or a metering pump. A gravimetric feeder feeds dry chemicals into the water by weight. A metering pump feeds a wet solution (a liquid) into the water by pumping a volume of solution with each stroke or rotation.
Improper coagulation related to coagulant may result from:
Using old chemicals
Using the wrong coagulant
Using the wrong concentration of coagulant.
This may result from setting the wrong feed rate on the gravimetric feeder or metering pump or from a malfunction of the equipment.
Review
Coagulation/flocculation is a process used to remove turbidity, color, and some bacteria from water.
In the flash mix chamber, chemicals are added to the water and mixed violently for less than a minute.
These coagulants consist of primary coagulants and/or coagulant aids.
Then, in the flocculation basin, the water is gently stirred for 30 to 45 minutes to give the chemicals time to act and to promote floc formation.
The floc then settles out in the sedimentation basin.
Coagulation removes colloids and suspended solids from the water.
These particles have a negative charge, so the positively charged coagulant chemicals neutralize them during coagulation.
Then, during flocculation, the particles are drawn together by van der Waal's forces, forming floc.
The coagulation/flocculation process is affected by pH, salts, alkalinity, turbidity, temperature, mixing, and coagulant chemicals.
| |
|
Admin
Admin

عدد المساهمات : 3762
تاريخ التسجيل : 15/09/2009
العمر : 57
الموقع : مصر
 |  موضوع: رد: Lمبادئ عملية التجلط والتطويف فى معالجة المياه/ Coagulation and Flocculation موضوع: رد: Lمبادئ عملية التجلط والتطويف فى معالجة المياه/ Coagulation and Flocculation  الأربعاء مارس 21, 2012 11:40 am الأربعاء مارس 21, 2012 11:40 am | |
| [ b]Location in the Treatment Plant
The primary purpose of the coagulation/flocculation process is the removal of turbidity from the water
three processes - flash mix, coagulation, and flocculation
Negatively charged particles repel each other due to electricity.
Positively charged coagulants attract to negatively
charged particles due to electricity.
Neutrally charged particles attract due to van der Waal's forces
Particles and coagulants join
together into floc.
Common Coagulation and Flocculation Problems [/b] [img]  [/img] [img]  [/img] [img]  [/img] [img]  [/img] [img]  [/img] [img]  [/img] [img]  [/img] [img]  [/img] [img] 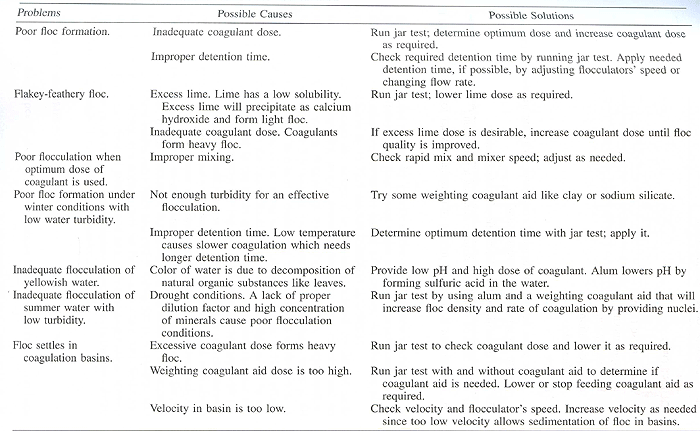 [/img] | |
|
Admin
Admin

عدد المساهمات : 3762
تاريخ التسجيل : 15/09/2009
العمر : 57
الموقع : مصر
 |  موضوع: العوامل المؤثرة على عملية التجلط والتطويف/The efficiency of the coagulation-flocculation process موضوع: العوامل المؤثرة على عملية التجلط والتطويف/The efficiency of the coagulation-flocculation process  الأربعاء مارس 21, 2012 11:54 am الأربعاء مارس 21, 2012 11:54 am | |
| The efficiency of the coagulation-flocculation process is dependent on many variables. For a particular water these may include:
§ Type of coagulant used
§ Coagulant dosage
§ Final pH
§ Coagulant feed concentration
§ Type and dosage of chemical additives other than primary coagulant (e.g. polymers)
§ Sequence of chemical addition and time lag between dosing points
§ Intensity and duration of mixing at rapid mix stage
§ Type of rapid mix device
§ Velocity gradients applied during flocculation stage
§ Flocculator retention time
§ Type of stirring device used
§ Flocculator geometry. | |
|








































![[MSF+3.jpg]](https://2img.net/h/4.bp.blogspot.com/_SynCnHGx75g/Shl2T3oVhsI/AAAAAAAAAEY/_7oem5pvQ3I/s1600/MSF%2B3.jpg)

















































