مجموعة تكنولاب البهاء جروب
تحاليل وتنقية ومعالجة المياه
|
تنظيف وتطهير وغسيل واعادة تاهيل الخزانات

معمل تكنولاب البهاء جروب
للتحاليل الكيميائية والطبية
والتشخيص بالنظائر المشعة
للمخدرات والهرمونات والسموم
وتحاليل المياه

مجموعة
تكنولاب البهاء جروب
لتصميم محطات الصرف الصناعى والصحى
لمعالجة مياه الصرف الصناعى والصحى
مجموعة تكنولاب البهاء جروب
المكتب الاستشارى العلمى
دراسات علمية كيميائية
معالجة الغلايات وانظمة البخار المكثف
معالجة ابراج التبريد المفتوحة
معالجة الشيللرات
مجموعة تكنولاب البهاء جروب
اسنشاريين
كيميائيين/طبيين/بكترولوجيين
عقيد دكتور
بهاء بدر الدين محمود
رئيس مجلس الادارة
استشاريون متخصصون فى مجال تحاليل وتنقية ومعالجة المياه
متخصصون فى تصنيع وتصميم كيماويات
معالجة الصرف الصناعى والصحى
حسب كل مشكلة كل على حدة تصنيع وتحضير كيماويات معالجة المياه الصناعية
مؤتمرات/اجتماعات/محاضرات/فريق عمل متميز     صور من وحدات معالجة المياه
 technolab el-bahaa group technolab el-bahaa groupTECHNOLAB EL-BAHAA GROUP
EGYPT
FOR
WATER
TREATMENT/PURIFICATION/ANALYSIS
CONSULTANTS
CHEMIST/PHYSICS/MICROBIOLIGIST
INDUSTRIAL WATER
WASTE WATER
DRINKING WATER
TANKS CLEANING
CHAIRMAN
COLONEL.DR
BAHAA BADR EL-DIN
0117156569
0129834104
0163793775
0174041455 تصميم وانشاء محطات صرف صناعى/waste water treatment plant design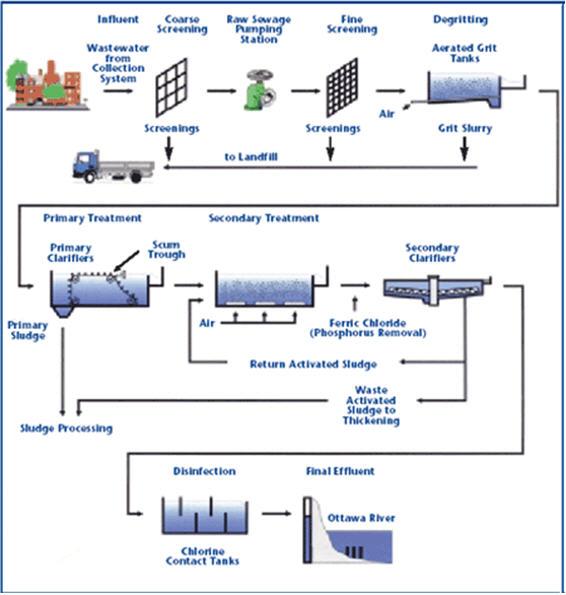 technolab el-bahaa group egypt We are a consultants in water treatment with our chemicals as:- Boiler water treatment chemicals Condensated steam treatment chemicals Oxygen scavenger treatment chemicals Ph-adjustment treatment chemicals Antiscale treatment chemicals Anticorrosion treatment chemicals Open cooling tower treatment chemicals Chillers treatment chemicals Waste water treatment chemicals Drinking water purification chemicals Swimming pool treatment chemicals Fuel oil improver(mazote/solar/benzene) technolab el-bahaa group
egypt
We are consultants in extraction ,analysis and trading the raw materials of mines as:-
Rock phosphate
32%-30%-28%-25%
Kaolin
Quartez-silica
Talcum
Feldspae(potash-sodumic)
Silica sand
Silica fume
Iron oxid ore
Manganese oxid
Cement(42.5%-32.5%)
Ferro manganese
Ferro manganese high carbon technolab el-bahaa group
web sites
e-mails
water treatment unit design 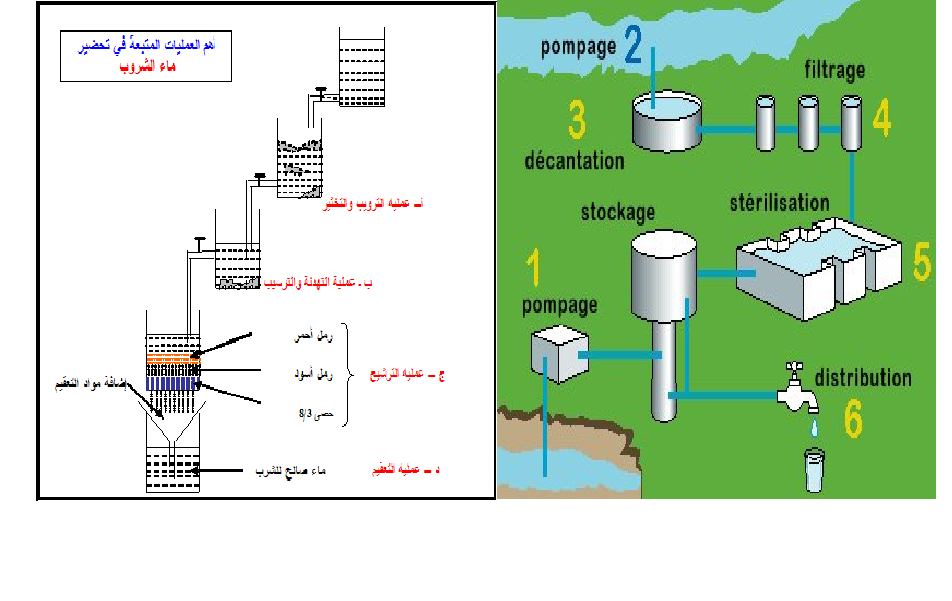
وكلاء لشركات تركية وصينية لتوريد وتركيب وصيانة الغلايات وملحقاتها
solo agent for turkish and chinese companies for boiler production/manufacture/maintance
وكلاء لشركات تركية وصينية واوروبية لتصنيع وتركيب وصيانة ابراج التبريد المفتوحة 
تصميم وتوريد وتركيب الشيللرات
design/production/maintance
chillers ابراج التبريد المفتوحة مجموعة تكنولاب البهاء جروب
المكتب الاستشارى العلمى
قطاع توريد خطوط انتاج المصانع
نحن طريقك لاختيار افضل خطوط الانتاج لمصنعكم
سابقة خبرتنا فى اختيار خطوط الانتاج لعملاؤنا
1)خطوط انتاج العصائر الطبيعية والمحفوظة والمربات
2)خطوط انتاج الزيوت الطبيعية والمحفوظة
3)خطوط انتاج اللبن الطبيعى والمحفوظ والمبستر والمجفف والبودرة
4)خطوط تعليب وتغليف الفاكهة والخضروات
5)خطوط انتاج المواسير البلاستيك والبى فى سى والبولى ايثيلين
6)خطوط انتاج التراى كالسيوم فوسفات والحبر الاسود
7)خطوط انتاج الاسفلت بانواعه
 محطات معالجة الصرف الصناعى والصحى بالطرق البيولوجية والكيميائية محطات معالجة الصرف الصناعى والصحى بالطرق البيولوجية والكيميائية9)محطات معالجة وتنقية مياه الشرب
10)محطات ازالة ملوحة البحار لاستخدامها فى الشرب والرى
11)الغلايات وخطوط انتاج البخار الساخن المكثف
12)الشيللرات وابراج التبريد المفتوحة وخطوط انتاج البخار البارد المكثف
للاستعلام
مجموعة تكنولاب البهاء جروب
0117156569
0129834104
0163793775
القاهرة-شارع صلاح سالم-عمارات العبور-عمارة 17 ب فلا تر رملية/كربونية/زلطيه/حديدية

وحدات سوفتنر لازالة عسر المياه

مواصفات مياه الشرب
Drinking water
acceptable
values
50 | colour | acceptable | Taste | nil | Odour | 6.5-9.2 | ph |
1 mg/dl | pb | 5 mg/dl | as | 50 mg/dl | cn | 10 mg/dl | cd | 0-100mg/dl | hg | 8 mg/dl | f | 45 mg/dl | N02 | 1 mg/dl | Fe | 5 mg/dl | Mn | 5.1 mg/dl | Cu | 200 mg/dl | Ca | 150 mg/dl | Mg | 600 mg/dl | Cl | 400 mg/dl | S04 | 200 mg/dl | Phenol | 15 mg/dl | zn |
الحدود المسموح به
ا لملوثات الصرف الصناعى
بعد المعالجة
Acceptable
values
treated wate water
|
7-9.5 | ph | 25-37 c | Temp | 40 mg/dl | Suspended solid | 35 mg/dl | bod | 3 mg/dl | Oil & grase | 0.1 mg/dl | hg | 0.02 mg/dl | cd | 0.1 mg/dl | cn | 0.5mg/dl | phenol | 1.5 ds/m | conductivity | 200 mg/dl | na | 120 mg/dl | ca | 56 mg/dl | mg | 30 mg/dl | k | 200 mg/dl | cl | 150 mg/dl | S02 | 0.75 mg/dl | Fe | 0.2 mg/dl | Zn | 0.5 mg/dl | Cu | 0.03 mg/dl | Ni | 0.09 mg/dl | Cr | 0.53 mg/dl | لb | 0.15 mg/dl | pb |
محطات تحلية مياه البحر بطريقة التقطير الومضى على مراحل MSF+3.jpg (image)محطات التقطير الومضى لتحلية مياه البحر2![[MSF+3.jpg]](https://2img.net/h/4.bp.blogspot.com/_SynCnHGx75g/Shl2T3oVhsI/AAAAAAAAAEY/_7oem5pvQ3I/s1600/MSF%2B3.jpg) some of types of tanks we services
انواع الخزانات التى يتم تنظيفها
ASME Specification Tanks
Fuel Tanks
Storage Tanks
Custom Tanks
Plastic Tanks
Tank Cleaning Equipment
Double Wall Tanks
Septic Tanks
Water Storage Tanks
Fiberglass Reinforced Plastic Tanks
Stainless Steel Tanks
Custom / Septic
مراحل المعالجة الاولية والثانوية والمتقدمة للصرف الصناعى

صور مختلفة
من وحدات وخزانات معالجة الصرف الصناعى
التى تم تصميمها وتركيبها من قبل المجموعة

صور
من خزانات الترسيب الكيميائى والفيزيائى
لوحدات معالجة الصرف الصناعى
المصممة من قبل المحموعة

 technolab el-bahaa group
 technolab el-bahaa group technolab el-bahaa group technolab el-bahaa group technolab el-bahaa group technolab el-bahaa group technolab el-bahaa group technolab el-bahaa group technolab el-bahaa group technolab el-bahaa group technolab el-bahaa group technolab el-bahaa group technolab el-bahaa group technolab el-bahaa group technolab el-bahaa group technolab el-bahaa group technolab el-bahaa group technolab el-bahaa group technolab el-bahaa groupمياه رادياتير اخضر اللون
بريستول تو ايه
انتاج شركة بريستول تو ايه - دمياط الجديدة
مجموعة تكنولاب البهاء جروب
اسطمبات عبوات منتجات شركة بريستول تو ايه-دمياط الجديدة مياه رادياتير خضراء فوسفورية من انتاج شركة بريستول تو ايه بترخيص من مجموعة تكنولاب البهاء جروب 


زيت فرامل وباكم DOT3 
|
|
| | دراسة جدوى فنية لتصنيع البطاريات (رصاص+حمض) النسخة الانجيليزية |  |
| | | كاتب الموضوع | رسالة |
|---|
Admin
Admin

عدد المساهمات : 3762
تاريخ التسجيل : 15/09/2009
العمر : 57
الموقع : مصر
 |  موضوع: دراسة جدوى فنية لتصنيع البطاريات (رصاص+حمض) النسخة الانجيليزية موضوع: دراسة جدوى فنية لتصنيع البطاريات (رصاص+حمض) النسخة الانجيليزية  الجمعة أكتوبر 19, 2012 8:59 am الجمعة أكتوبر 19, 2012 8:59 am | |
|
ADVANCED LEAD-ACID BATERIES
by
technolab el-bahaa group
general.dr
bahaa badr
01229834104
Lead-acid batteries are used in automotive electrical systems, and some information about their production is available.
They are also recycled commercially.
The production and recycling processes for materials in conventional lead-acid batteries can be used with minimal changes for advanced EV batteries
Therefore, materials energy use, and emissions from the manufacture and recycling of these batteries can be estimated The battery lifetime is up to 80,000 miles, so one replacement would generally be necessary over an EV's lifetime
---------------------------------------------------------------------------------------
BATTERY DESIGN-
The Horizon EV battery developed by Electro source (Austin, Texas) and BDM Technologies, is an advanced lead-acid battery slated to begin limited commercial production in 1995.
This battery has an energy density of over 45 Whkg.
It uses electrodes produced by co-extruding "an alloy of lead onto a high tensile strength core material, making a small diameter dimensionally stable wire that is woven into lightweight bipolar mesh grids
The core can be graphite or fiberglass
The electrodes are then coated with a proprietary paste and assembled horizontally into batteries
The paste is reportedly made from refined lead dust and dilute sulfuric acid.
For the negative electrodes, a small amount (4%o)f carbon black, barium sulfate, and lignin sulfonate is added.
For the positive, about 0.03% of a polymer, such as polypropylene (PP), or possibly glass fiber, Is added
Fiberglass mats between the porous electrodes separate them and hold the acid
electrolyte which is all absorbed in the mats and electrodes
The manufacturing equipment for these batteries is being developed, but the infrastructure already in-place can be used to recycle them.
Over 98% of spent auto batteries in the U.S. are currently recycled.
-------------------------------------------------------------------------------------
BATTERY MATERIALS-
Because even advanced Pb-acid batteries have the lowest energy density of the batteries considered, they use the largest mass of materials.
At 50 Wh/kg, a 25-kWh battery module would weigh 500 kg (1100 Ib).
The materials inside advanced lead-acid batteries are lead, lead oxide, acid, and fiberglass mats
The containers covers are generally PP, but steel'can also be used.
Aluminum trays were considered, but lightweight steel was determined to be cheaper m- Energy required to produce primary lead was earlier estimated to be roughly 23.4 x 1 0' Btu/ton.
(Note Btu/ton = 1.16 MJ/kg.)
The same source estimated secondary lead production to require 9.5 x 10' Btu/ton compared to a more recent estimate of 4.6 x 1 0' Btu/ton for the smelting step alone at a modern smelter.
We expect that primary production energy also has been reduced
Primary lead is produced from sulfide ores by sintering, reduction in a blast furnace, and refining to purity.
99.99 primary effluent is SO,, nearly 85% of which is produced
during sintering.
Half of the remainder is captured in the+% As is typical for smelting of sulfide ores, the
slag; most of the rest is recovered from the blast furnace Most of the impurities are entrained in the slag.
In addition lead compounds, such as oxides, are released as particulates during both primary and secondary (recycling lead smelting operations, and during battery manufacture and recycling.
Sulfur oxides and lead are both serious health and environmental concerns; regulations in the U.S have generally resulted in careful control of these emissions
.
(The EPA has forced older plants not meeting these standards to close.) Particulates are usually controlled with a baghouse, with control efficiencies exceeding 99%.
Sulfur dioxide is recovered and converted to sulfuric acid (see next section).
Emission factors reported for primary lead smelting are lead, 0.034 kg/tonne (0.07 Ib/t), and uncontrolled SO,, 23 kg/tonne (45 Ib/t).
Controlled lead emissions from secondary smelting are approximately
0.1 5 kg/tonne (0.29 IWt), and uncontrolled SO, emissions,
40 kg/tonne (80 IWt), but these values are based on very
limited data. m Fugitive emissions may be somewhat
higher; no actual data are available.
The fuel heductant in the blast furnace is metallurgical coke (8-14% of the charge); emissions from coke production are not discussed
Secondary smelting and battery recycling are more geographically spread and more likely to occur near population centers Solid wastes generated from mining operations remain in somewhat remote locations.
Slag produced during smelting is a relatively inert solid that is generally disposed of
--------------------------------------------------------------------------------------
Sulfuric Acid-
The electrolyte in lead-acid batteries is
22-26% sulfuric acid (H2S0,J, generally made by
combustion of sulfur to sulfur dioxide (SOJ,
further oxidation to sulfur trioxide using a vanadium-based catalyst and absorption into strong sulfuric acid followed by dilution to the desired concentration.
,
Alternatively, by-product SO, from smelting of sulfide ores (e.g., lead sulfide) can be the raw material.
In either case, the reactions are highly exothermic and fuel purchases are generally not required (except small quantities of natural gas during startup).
The tail gas from the final absorption tower may contain small quantities of SO,, and even smaller quantities of SO,.
Older single-absorption plants can achieve emission levels of 27 IWt H,SO, (97.9% conversion), and newer dual-absorption plants can meet the 4-lb/ton NSPS conversion).
99.7 H,SO, mist from the stack is generally
controlled by demister pads or mist eliminators that reduce emissions by more than 95
%.
Sulfuric acid is generally produced where metals are smelted from sulfide ores, well outside population centers Acid mist is also produced during battery-breaking
(recycling)
--------------------------------------------------------------------------------------
Polypropylene
is a polymer of the organic chemical propylene (C,H,), a co-product of ethylene manufacture from natural gas liquids or petroleum Energy required for PP production is approximately 68 X lO'BWton, mostly in the form of oil and gas
Recycling simply requires remelting at low temperatures, and therefore requires considerably less energy, on the order of x 10' Btulton
Emissions from propylene polymerization, shown in Table 1, are in the form of particulates (polymer resin) and gases (mostly the propylene monomer).
Pollution control is via the systems for recovery of reactants or products.
Emissions from the entire PP production chain (not included here) occur at petroleum refineries or natural gas processing plants and at large chemical-production complexes.
-------------------------------------------------------------------------------------
fiberglass
- The production of fiberglass is very similar to that of container or float glass (sheet), except for the final fabrication step.
The raw materials (mainly sand limestone, and soda ash) are transported to a plant, where they are melted at high temperature and then formed into fibers.
Energy required to produce fiberglass, including energy to produce and transport the chemical inputs, is estimated to be 22.3 x lo6 BtWton, mostly in the form of natural gas.
Energy to recycle would be somewhat lower about 18.9 x 1 O6 Btu/tonl but it is not clear that sufficiently clean material could be separated from battery scrap Fugitive dust and raw-material particles from raw material handling are controlled by moist handling or by fabric filters on enclosed transfer points
Emissions from melting and refining may include volatile organics, raw material particles, and combustion gases; these are controlled by fabric filters.
Particulates are the main emissions from fiber-forming.
Particulate emissions are common to all glass manufacture; boron and fluoride emissions result from the special chemical composition of fiberglass.
-----------------------------------------------------------------------------------------
RECYCLING PATHS-
The electrode grids from spent lead-acid batteries are often corroded and stretched out so that electrical contact is reduced or lost.
Lead sulfate is formed in crystals large enough that they do not reconvert during charging.
Therefore, the battery components cannot simply be reused; they must be reprocessed.
Currently over 90% of the lead and lead oxides from batteries is recycled or exported for recycling.
lead supply, but some uses require the purer primary product.
Approximately 80% of secondary lead is used to produce new automotive batteries Currently, the price of imported lead is low, presenting an incentive to import lead and export scrap instead of recycling it S.
If scrap is exported to Asia, smelters operating there with less stringent (or no) pollution-control regulations could have an economic advantage and cause severe health effects.
Another barrier to increased lead recycling is that secondary slag is categorized as hazardous by the EPA, but primary slag, chemically the same material, is not.
Transportation and recycling of spent lead-acid batteries is also regulated.
This is typical of the U.S. regulatory system In an operating lead-acid-battery recycling plant, the batteries are dismantled mechanically.
.
The top of the battery is sheared off and the acid drained.
The lead is separated out as metallic, oxide, and sulfate fractions and recycled to new electrodes.
The casings and plate separators are segregated; the PP case fragments are recovered by a sinklfloat process and recycled to new battery cases or other products, like plastic piping.
The acid is cleaned by solvent extraction and reused, or it can be used to neutralize KOH or to make sodium sulfate crystals for soap manufacture.
The glass mats from advanced leadacid batteries reportedly can be recycled
Plant emissions are controlled to meet EPA and OSHA regulations; the plant discharge contains less than 200 ppm SO
Processes to recover lead from the sulfates (and reduce sulfur emissions) are under development by the U.S. Bureau of Mines.
Paste containing lead sulfate and lead oxide can be desulfurized with soda ash to produce marketable sodium sulfate; the desulfurized paste can then be processed in a reverberatory furnace or electrowon.
Electrowinning could be used to improve lead recovery rates and produce a higher-purity product.
R&D on this process is ongoing; recent papers include detailed process descriptions and estimates of energy use for secondary ead recovery by electrowinning.
lThe energy required is approximately 6.7 x lo6 Btu/ton, somewhat higher than that required by secondary smelting. [l 11 However, this process recovers material not easily treated in a secondary smelter Lead emissions from pyrometallurgical processes are likely to exceed those from hydrometallurgical processes.
.
| |
|   | | Admin
Admin

عدد المساهمات : 3762
تاريخ التسجيل : 15/09/2009
العمر : 57
الموقع : مصر
 |  موضوع: معلومات عامة عن بطارية السيارة ومشاكلها (مقدمة الفصل الاول) النسخة الانجيليزية موضوع: معلومات عامة عن بطارية السيارة ومشاكلها (مقدمة الفصل الاول) النسخة الانجيليزية  الجمعة أكتوبر 19, 2012 9:31 am الجمعة أكتوبر 19, 2012 9:31 am | |
|
Car Battery Information
مجموعة تكنولاب البهاء جروب
01229834104
Car Battery Shop's lead acid car batteries are built to the highest standards.
They are manufactured, in most cases, to correspond with car manufacturer’s requirements and specifications.
Nevertheless, it is important to understand that:-
A wet car battery is ‘alive’. Whether it is in service or in storage it has a finite life span.
If stored in a wet (filled) condition all batteries will slowly self-discharge.
The higher the ambient (storage) temperature the greater the rate of self-discharge.
To ensure that car batteries are not allowed to discharge to the point where they are either damaged (sulphated) or so that they are incapable of starting the car or operating equipment, regular voltage checks should be made (monthly).
Car batteries with a voltage of 12.4v or below should be recharged. Recharging must not be effected by the means of a rapid charger.
Ideally a recharge rate of 1/10th of the battery’s capacity should be applied for up to 12 hours.
At the end of this period, a fully charged car battery will read over 12.65v and all cells should be gassing freely.
Remember if a battery has vent plugs, these should always be removed before charging.
========================================================
Car Battery problems - Non-manufacturing faults
Sulphation of a car battery:
If a car battery is allowed to stand in a discharged state for an excessive amount of time, a chemical reaction takes place, which can permanently impair performance - this is sulphation.
Sulphation can be seen as a fine white/grey coating on the plates.
In most cases this signifies irreversible damage and the car battery will not be serviceable. This damage can occur either in storage or if the car battery is installed in a vehicle (or equipment) that is not used for a period of time, for example a tractor, motorcycle, or boat.
Even a car or truck that is stored with the battery connected can still damage the battery in this way.
This is because there is a permanent drain on the battery from the clock, alarm etc.
As a result the level of charge in a car battery falls, and after a period of time sulphation will build up on the plates.
The sulphation (lead sulphate) hinders the chemical reaction between the acid (electrolyte) and the active mass (lead compound) in the plates and prevents the car battery from operating as normal. This is not a manufacturing fault.
=======================================================
Wear and Tear of a car battery:
During the charge and discharge cycle, material from the battery plates (active mass) is in motion, through the electrochemical reaction that produces electricity.
Every time the car battery goes through a charge and discharge cycle, a small amount of the active mass is lost from the plates.
Because the ultimate life of a car battery depends on so many factors, it is impossible to stipulate a minimum/maximum life expectancy.
This process of normal ageing through the charge and discharge cycle will eventually cause the car battery to lose capacity, and it will come to the point where the battery can no longer start the vehicle/equipment. This is not a manufacturing fault.
A car battery only has a finite number of cycles (x) it can go through before it loses its capacity to perform.
Vehicles with high usage such as taxi’s, minicabs, trucks, and buses will often subject the battery to its x number of cycles but over a much shorter time. As a result, batteries on these vehicles can display the above symptoms after 12-24 months. This is not a manufacturing fault.
======================================================
Deep cycling a car battery:
As mentioned above, every time a car battery goes through a charge and discharge cycle a small amount of the material from the plates is lost.
If a car battery is subjected to deep discharging (i.e. over 40%) and then rapid charging, this process is accelerated.
Additionally, if during the recharge the car battery is not adequately compensated for the discharge cycle, the battery will quickly exhibit loss of performance.
Even after recharging the voltage will be low (under 12.4v) but the cells will generally give even readings. This is not a manufacturing fault.
Overcharging a car battery:If the regulator is not set properly, then the car battery can be subjected to an excessive charge.
If left unchecked the battery will overheat and will start to evaporate the electrolyte.
The overcharging will cause the accelerated break up of the active mass on the plates and the car battery will lose performance.
This is generally obvious from the examination of the battery - the acid levels will be very low, and quite often a black coating will be visible on the filler caps. This is not a manufacturing fault.
=========================================================
Physical damage to a car battery:
If the car battery is fitted incorrectly, if the connector leads are hammered onto the terminals, or if the leads are not properly fastened, the car battery will have obvious damage to the casing or the terminals. This is not a manufacturing fault.
========================================================
Incorrect application of a car battery:
The car batteries recommended by Car Battery Shop are those equal to or above the original equipment specification. Fitting a smaller or less powerful car battery will result in a shorter service life and earlier failure, which will generally manifest itself as deep cycling/premature wear and tear. This is not a manufacturing fault.
========================================================
Car battery problems - Manufacturing faults
Due to the high demands of the OEM market, and taking into account the technical and manufacturing standards adhered to by Car Battery Shop, the rate of genuine manufacturing faults is negligible.
========================================================
Car battery short circuit/dead cell:
Typically seen in a car battery with a short (under 12 months) service life.
One cell will show a dramatically lower specific gravity (SG) reading than the others.
The affected cell will boil visibly under a high rate discharge test. In some cases it may also be visible as a sulphated cell (see above).
The remaining cells will show a good SG reading of 1.26 or over.
Internal Break in a car battery: The car battery will have good SG readings but no voltage.
Summary
Provided the right car battery, in the right condition is used for the right application, the number of battery problems encountered will be minimum.
All car batteries have a finite life span, which is governed by the conditions under which the battery operates.
Car battery failures caused by sulphation, wear and tear, or deep cycling are not manufacturing faults and are not covered by a guarantee.
It is important to remember that under normal operating conditions, a car battery cannot become discharged on its own. The reason for this discharge is normally attributable to:-
Malfunctioning alternator, regulator, or starter motor
Slipping fan belt
Electrical fault
Excessive use of electrical consumers - car phones, air conditioning etc
Long standing time without recharge
Boot light/glove box malfunction
Vehicle lights being left on
If a car battery is consistently used/left in a discharged condition, it will eventually reach a condition where even a prolonged recharge will not return to its original condition. This is classified as deep discharge/undercharging and is not a manufacturing fault.
If a car battery is continuously deeply discharged by, for example, stop start motoring and heavy use of the car phone, air conditioning etc, and is then not adequately recharged, it will lose its performance relatively quickly. This is called deep cycling/wear and tear and is not a manufacturing fault.
| |
|   | | Admin
Admin

عدد المساهمات : 3762
تاريخ التسجيل : 15/09/2009
العمر : 57
الموقع : مصر
 |  موضوع: رد: دراسة جدوى فنية لتصنيع البطاريات (رصاص+حمض) النسخة الانجيليزية موضوع: رد: دراسة جدوى فنية لتصنيع البطاريات (رصاص+حمض) النسخة الانجيليزية  الأحد أكتوبر 21, 2012 4:07 pm الأحد أكتوبر 21, 2012 4:07 pm | |
| | |
|   | | Admin
Admin

عدد المساهمات : 3762
تاريخ التسجيل : 15/09/2009
العمر : 57
الموقع : مصر
 | |   | | Admin
Admin

عدد المساهمات : 3762
تاريخ التسجيل : 15/09/2009
العمر : 57
الموقع : مصر
 |  موضوع: رد: دراسة جدوى فنية لتصنيع البطاريات (رصاص+حمض) النسخة الانجيليزية موضوع: رد: دراسة جدوى فنية لتصنيع البطاريات (رصاص+حمض) النسخة الانجيليزية  الخميس أكتوبر 25, 2012 3:25 am الخميس أكتوبر 25, 2012 3:25 am | |
| Construction
Plates
The lead–acid cell can be demonstrated using sheet lead plates for the two electrodes.
However such a construction produces only around one ampere for roughly postcard sized plates, and for only a few minutes.
Gaston Planté found a way to provide a much larger effective surface area.
In Planté's design, the positive and negative plates were formed of two spirals of lead foil, separated with a sheet of cloth and coiled up.
The cells initially had low capacity, so a slow process of "forming" was required to corrode the lead foils, creating lead dioxide on the plates and roughening them to increase surface area.
Initially this process used electricity from primary batteries; when generators became available after 1870, the cost of production of batteries greatly declined.
Planté plates are still used in some stationary applications, where the plates are mechanically grooved to increase their surface area.
Faure pasted-plate construction is typical of automotive batteries.
Each plate consists of a rectangular lead grid alloyed with antimony or calcium to improve the mechanical characteristics.
The holes of the grid are filled with a paste of red lead and 33% dilute sulfuric acid. (Different manufacturers vary the mixture).
The paste is pressed into the holes in the grid which are slightly tapered on both sides to better retain the paste.
This porous paste allows the acid to react with the lead inside the plate, increasing the surface area many fold.
Once dry, the plates are stacked with suitable separators and inserted in the battery container.
An odd number of plates is usually used, with one more negative plate than positive. Each alternate plate is connected.
The positive plates are the chocolate brown color of lead dioxide, and the negative are the slate gray of "spongy" lead at the time of manufacture. In this charged state the plates are called 'formed'.
One of the problems with the plates is that the plates increase in size as the active material absorbs sulfate from the acid during discharge, and decrease as they give up the sulfate during charging.
This causes the plates to gradually shed the paste.
It is important that there is room underneath the plates to catch this shed material.
If it reaches the plates, the cell short-circuits.
The paste contains carbon black, blanc fixe (barium sulfate) and lignosulfonate.
The blanc fixe acts as a seed crystal for the lead–to–lead sulfate reaction.
The blanc fixe must be fully dispersed in the paste in order for it to be effective.
The lignosulfonate prevents the negative plate from forming a solid mass during the discharge cycle, instead enabling the formation of long needle–like crystals.
The long crystals have more surface area and are easily converted back to the original state on charging.
Carbon black counteracts the effect of inhibiting formation caused by the lignosulfonates.
Sulfonated naphthalene condensate dispersant is a more effective expander than lignosulfonate and speeds up formation.
This dispersant improves dispersion of barium sulfate in the paste, reduces hydroset time, produces a more breakage-resistant plate, reduces fine lead particles and thereby improves handling and pasting characteristics.
It extends battery life by increasing end–of–charge voltage.
Sulfonated naphthalene requires about one-third to one-half the amount of lignosulfonate and is stable to higher temperatures.
Practical cells are usually not made with pure lead but have small amounts of antimony, tin, calcium or selenium alloyed in the plate material to add strength and simplify manufacture.
The alloying element has a great effect on the life of the batteries, with calcium-alloyed plates preferred over antimony for longer life and less water consumption on each charge/discharge cycle.
About 60% of the weight of an automotive-type lead–acid battery rated around 60 Ah (8.7 kg of a 14.5 kg battery) is lead or internal parts made of lead; the balance is electrolyte, separators, and the case.
Separators
Separators between the positive and negative plates prevent short-circuit through physical contact, mostly through dendrites (‘treeing’), but also through shedding of the active material.
Separators obstruct the flow of ions between the plates and increase the internal resistance of the cell. Wood, rubber, glass fiber mat, cellulose, and PVC or polyethylene plastic have been used to make separators.
Wood was the original choice, but deteriorated in the acid electrolyte. Rubber separators were stable in the battery acid.
An effective separator must possess a number of mechanical properties; such as permeability, porosity, pore size distribution, specific surface area, mechanical design and strength, electrical resistance, ionic conductivity, and chemical compatibility with the electrolyte.
In service, the separator must have good resistance to acid and oxidation.
The area of the separator must be a little larger than the area of the plates to prevent material shorting between the plates.
The separators must remain stable over the battery's operating temperature range. | |
|   | | Admin
Admin

عدد المساهمات : 3762
تاريخ التسجيل : 15/09/2009
العمر : 57
الموقع : مصر
 |  موضوع: رد: دراسة جدوى فنية لتصنيع البطاريات (رصاص+حمض) النسخة الانجيليزية موضوع: رد: دراسة جدوى فنية لتصنيع البطاريات (رصاص+حمض) النسخة الانجيليزية  الخميس أكتوبر 25, 2012 3:31 am الخميس أكتوبر 25, 2012 3:31 am | |
| Sulfation and desulfation
Lead–acid batteries lose the ability to accept a charge when discharged for too long due to sulfation, the crystallization of lead sulfate.
They generate electricity through a double sulfate chemical reaction.
Lead and lead dioxide, the active materials on the battery's plates, react with sulfuric acid in the electrolyte to form lead sulfate.
The lead sulfate first forms in a finely divided, amorphous state, and easily reverts to lead, lead dioxide and sulfuric acid when the battery recharges.
As batteries cycle through numerous discharges and charges, some lead sulfate is not recombined into electrolyte and slowly converts to a stable crystalline form that no longer dissolves on recharging.
Thus, not all the lead is returned to the battery plates, and the amount of usable active material necessary for electricity generation declines over time.
Sulfation occurs in all lead–acid batteries during normal operation.
It impedes recharging; sulfate deposits ultimately expand, cracking the plates and destroying the battery.
Eventually so much of the battery plate area is unable to supply current that the battery capacity is greatly reduced.
In addition, the sulfate portion (of the lead sulfate) is not returned to the electrolyte as sulfuric acid.
The large crystals physically block the electrolyte from entering the pores of the plates. Sulfation can be avoided if the battery is fully recharged immediately after a discharge cycle.
A white coating on the plates may be visible (in batteries with clear cases, or after dismantling the battery).
Batteries that are sulfated show a high internal resistance and can deliver only a small fraction of normal discharge current.
Sulfation also affects the charging cycle, resulting in longer charging times, less efficient and incomplete charging, and higher battery temperatures.
The process can often be at least partially reversed by a desulfation technique called pulse conditioning, in which short but powerful current surges are repeatedly sent through the damaged battery.
Over time, this procedure tends to break down and dissolve the sulfate crystals, restoring some capacity.
Desulfation is the process of reversing the sulfation of a lead-acid battery.
Desulfation is achieved by high current pulses produced between the terminals of the battery.
This technique, also called pulse conditioning, breaks down the sulfate crystals that are formed on the battery plates.
Short high current pulses tend to work best.
Electronic circuits are used to regulate the pulses of different widths and frequency of high current pulses.
These can also be used to automate the process since it takes a long period of time to desulfate a battery fully.
Battery chargers designed for desulfating lead-acid batteries are commercially available.
A battery will be unrecoverable if the active material has been lost from the plates, or if the plates are bent due to over temperature or over charging.
Batteries which have sat unused for long periods of time can be prime candidates for desulfation.
A long period of self-discharge allows the sulfate crystals to form and become very large.
Some typical cases where lead acid batteries are not used frequently enough are planes, boats (esp sail boats), old cars, and home power systems with battery banks that are under utilized.
Some charging techniques can aid in prevention such as equalization charging and cycles through discharging and charging regularly.
It is recommended to follow battery manufacturer instructions for proper charging.
SLI batteries (starting, lighting, ignition; i.e. car batteries) have less deterioration because they are used more frequently vs deep cycle batteries.
Deep cycle batteries tend to require more desulfation, can suffer from overcharging, and can be in a very large bank which leads to unequal charging and discharging. | |
|   | | | | دراسة جدوى فنية لتصنيع البطاريات (رصاص+حمض) النسخة الانجيليزية |  |
|
مواضيع مماثلة |  |
|
| | صلاحيات هذا المنتدى: | لاتستطيع الرد على المواضيع في هذا المنتدى
| |
| |
| |
|








































![[MSF+3.jpg]](https://2img.net/h/4.bp.blogspot.com/_SynCnHGx75g/Shl2T3oVhsI/AAAAAAAAAEY/_7oem5pvQ3I/s1600/MSF%2B3.jpg)










































